Acid and Base Reaction
Group: Qiaoyang, Junyi, Shaomiao, Xinyi L.
Overview
The lesson introduces the chemical concepts of acids and bases, which are essential in chemistry and valuable in real life. In chemistry, it is the basis of many studies, and researchers and chemists do many experiments that are conducted in solutions that are either acids, bases, or neutral. In our daily life, acids and bases comprise many of our daily uses. Learning about acids and bases could help us better understand the substances we use daily and avoid possible misuse that could lead to harmful consequences.
Lesson Objectives
Learners will be able to
- Understand and tell what particles make the solution an acid or base.
- Identify whether a solution is acid, base, or mutual after knowing what’s in the solution.
- Tell the standard way to test whether a solution provided is acidic or basic.
Read/Watch
Read: Chemical Properties of Acids and Bases (10:00)
Watch: Acid-Base Theories (5:38)
Content
Solution
What is a solution in chemistry? In simple words, a solution is where two or more substances are mixed. The state of a solution we usually talk about is liquid, but could also be gas or solid.
The above picture shows a liquid solution. The mixtures of the two substances are solute and solvent (the solvent is usually water).
Acid
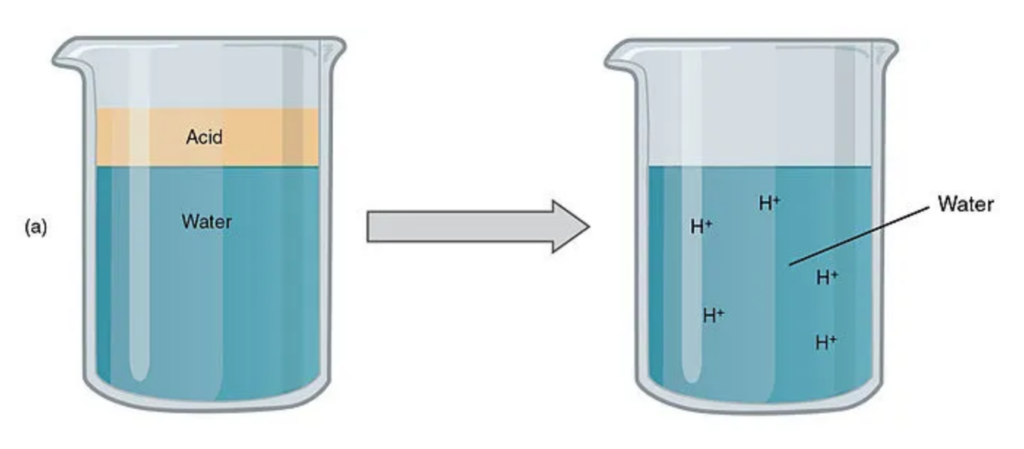
When some substances dilute in water, their molecules break apart and produce H+. This ion H+ makes the solution show properties of an acid that
- are sour in taste.
- furnish hydrogen ions in an aqueous solution.
- reacts with the metal to form hydrogen gas.
- reacts with carbonates and liberates carbon dioxide gas.
- litmus turns red in acid.
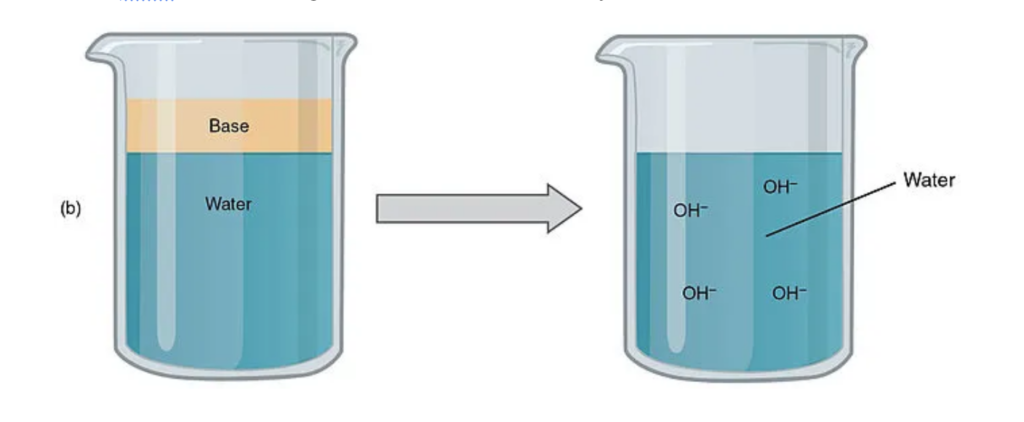
When some substances dilute in water, their molecules break apart and produce OH_. This ion OH_ makes the solution show properties of a base that
- are bitter in taste.
- loses its basicity when mixed with acids.
- reacts with acids to form salt and water. …
- can conduct electricity.
- feels slippery or soapy.
- some of them are great conductors of electricity.
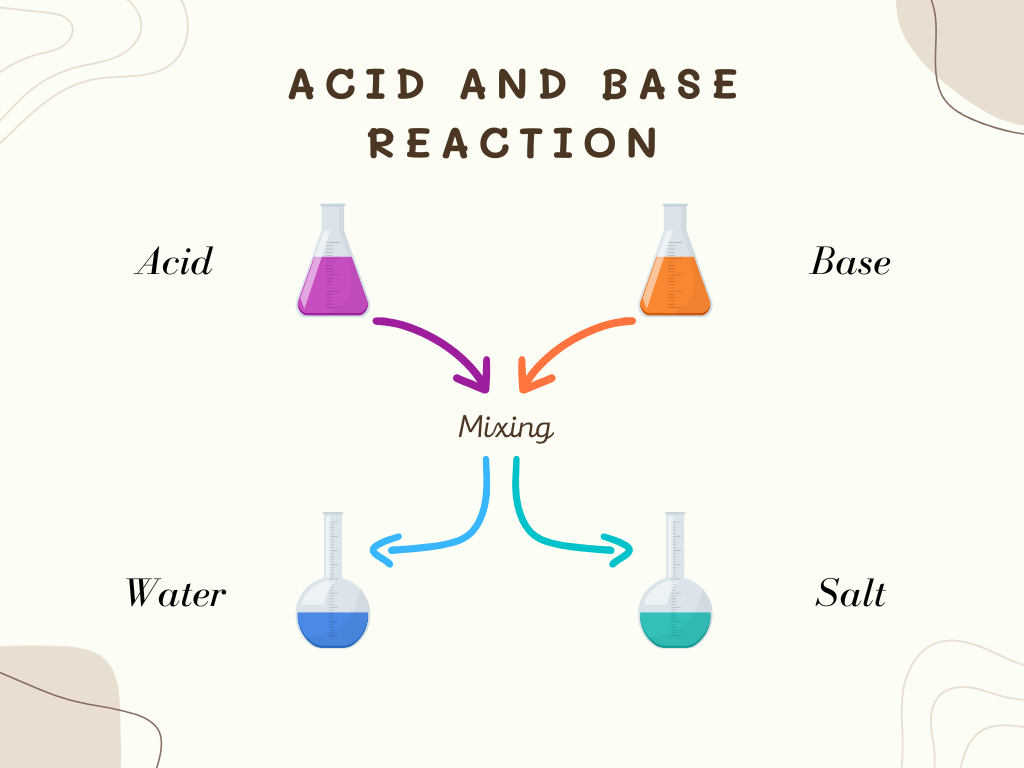
Reaction
When acid and base solutions are mixed, the H+ in an acid and OH_ in a base react and become H2O which is water. If the amount of H+ and OH_ are the same, the mixture of the acid and base solution neutralizes.
pH Scale
It leads us to how we can tell whether a solution is acidic, basic, or neutral. One common way is to use litmus paper to test a solution. The solution is acidic if the paper turns red and basic if blue.
Here’s a picture of litmus paper
ACIDS AND BASES SONG | Science Music Video, shows the testing of acid and base and the chemistry behind it to familiarize you with the concepts.
Practice
Application
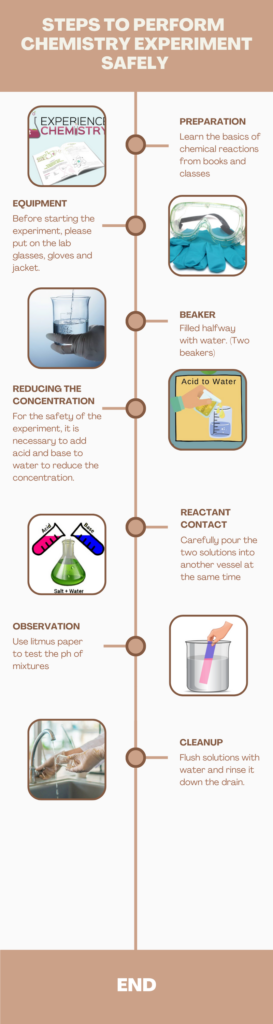
After learning the basics of acids and bases, students can verify what they have learned in the lab. Acids and Bases Experiments Student Handout
Here are the basic steps for performing a chemistry experiment in the laboratory.
Reflection
Based on the knowledge of acid-base reactions, can you explain why a solvent mixture of vinegar and baking soda can be used as a good kitchen cleaner?
Theories and Principles
- Dual Coding Theory: Whenever possible, we use text and images to describe the content. The use of visual aids can reduce the Cognitive Load of the reader to some extent.
- Signaling principle: We use the heading to remind each section of the topic’s content.
- Segmenting principle: We have divided the content related to acid-base reactions into several parts, such as the basic properties of acids and bases, the principles of reactions, and the application of ph values.
- Redundancy Principle: To prevent an extraneous load on the reader, we have kept unnecessary over-descriptions to a minimum.
References
Madhu, “Difference between acid and base,” Compare the Difference Between Similar Terms, 26-Apr-2018. [Online]. Available: https://www.differencebetween.com/difference-between-acid-and-vs-base/. [Accessed: 03-Dec-2022].
Studypug, “Introduction to solution chemistry and solubility.” [Online]. Available: https://www.studypug.com/chemistry-help/intro-to-solution-chemistry-and-solubility. [Accessed: 03-Dec-2022].
Toppr, “Chemical properties of acids and bases.” [Online]. Available: https://www.toppr.com/guides/chemistry/acids-bases-and-salts/chemical-properties-of-acids-and-bases/. [Accessed: 03-Dec-2022].
Lab Manager, “Lab safety rules and guidelines,” Lab Manager. [Online]. Available: https://www.labmanager.com/lab-health-and-safety/science-laboratory-safety-rules-guidelines-5727. [Accessed: 03-Dec-2022].
R. E. Mayer, “Principles for Reducing Extraneous Processing in Multimedia Learning: Coherence, Signaling, Redundancy, Spatial Contiguity, and Temporal Contiguity Principles,” in The Cambridge Handbook of Multimedia Learning, New York, NY: Cambridge Univ. Press, 2014, pp. 279–315.
R. P. Bell, “Acid–base reaction,” Encyclopædia Britannica. [Online]. Available: https://www.britannica.com/science/acid-base-reaction. [Accessed: 03-Dec-2022].